Views: 0 Author: Allison Scott, MicronView Publish Time: 2022-09-13 Origin: Site

A new revision of the EU GMP Annex 1 was released by the European Commission in August of 2022. This long-awaited revision replaces that released in 2008 and comes two years after the release of the most recent draft version of the document. The regulatory standard for manufacture of sterile medicinal products has increased in length from 16 to 59 pages, now including ten sections, new requirements, and significantly more detail. The industry has one year to implement, with a deadline for coming into operation on the 25th of August 2023 for all but section 8.123, which has been given two years. One reason listed for the updates is to clarify how manufacturers can take advantage of new possibilities deriving from the application of an enhanced process understanding by using innovative tools as described in the ICH Q9 and Q10 guidelines.
Quality risk management (QRM) and contamination control strategy (CCS) are mentioned throughout the document in support of proactively identifying, evaluating and controlling risks to quality, and working to ensure prevention of contamination in the final product. There is a focus on increased process awareness (e.g., through continuous monitoring and trending) and control (e.g., through use of isolators and restricted access barrier systems (RABS)). With regards to the latter, it is mentioned that RABS and isolators should be considered and any alternative to their use should be justified. This is a significant progression from the previous revision of Annex 1, which mentioned that these technologies may be beneficial. The new revision also includes significant mention of alternative approaches, rapid and alternative methods, continuous monitoring and trend analysis. Note that of all these terms, quality risk management, isolators and RABS are the only terms that appeared in the 2008 revision.
This is a welcome change given the fourteen years since the previous revision was released, which contained no mention of such technologies. Continuous total particle and viable monitoring is also recommended, primarily for Grade A, and a significant departure from the previous revision. Trending is also stressed throughout the document to support QRM, a timely identification of adverse trend or a change in the environment, and potential for faster resolution if identified, which is highly relevant to use of MICRONVIEW' BAMS, WBMS and BAS (BAMS, BAS).
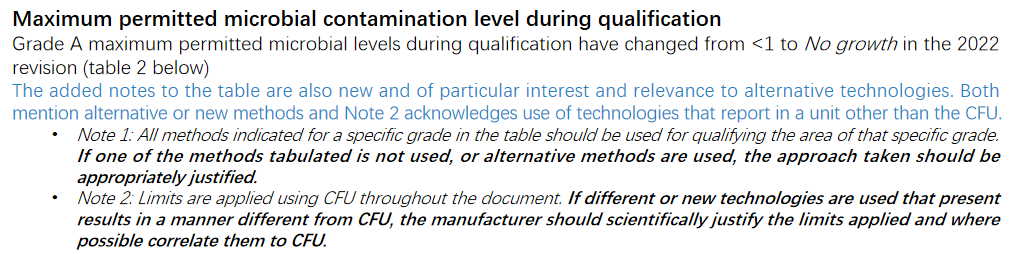
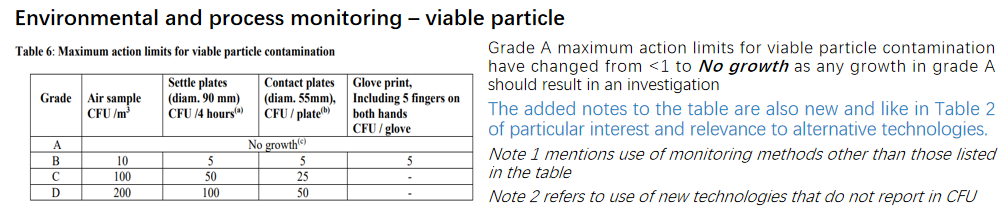
Significantly more detail is supplied throughout the document, including forward thinking acknowledgment of alternative technologies, the need for continuous monitoring, and benefits of trend analysis. Although microbial contamination levels are still listed in terms of the colony forming unit (CFU) and technologies based on the culture method, use of alternative technologies that report in a unit other than the CFU are acknowledged in a note to Table 2: Maximum permitted microbial contamination level during qualification and Table 6: Maximum action limits for viable particle contamination. In a departure from the previous revision, maximum microbial contamination levels have been separated into these two tables for qualification and monitoring, in section 4 and section 9 of the document. Total particle limits for classification and monitoring have also been separated into Table 1 and Table 5 in these two sections.
Many exciting and progressive updates have been made to this long-anticipated revision of Annex 1. The next year will prove insightful in terms of the incorporation of this updated guidance in operation.
Learn more about what is the history, changes, and next steps refer to EU GMP Annex 1 please join in upcoming free live course via below link:
https://www.micronview.com/News-and-Events-ic251568.html
